 400-028-6288
400-028-6288 在线客服
在线客服
 官方微信
官方微信
 400-028-6288
400-028-6288 在线客服
在线客服
 官方微信
官方微信

新闻动态
苯酚作为一个重要的药物中间体,可以转化为许多官能团。对它的合成研究有许多,如前段时间,给大家分享了许多苯酚的合成方法:
这次,小编查阅相关文献发现了苯酚合成的另一方法,特分享给大家。采用芳基卤素直接合成酚的方法:以Pd/RockPhos为催化剂,苯甲醛肟为OH源,合成得到苯酚。
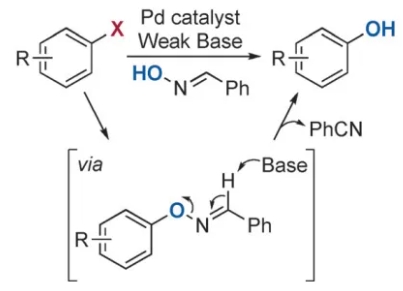
实施例:无论供电基团还是吸电基团的芳基卤都能高收率得到苯酚,羰基化合物,氰基,吲哚等都耐受,其次杂环芳基也能轻松得到苯酚,简直不要太爽。溶解度较差的喹啉环,活性较低的芳基氯也能也能成功得到。
药物中的应用:可以看出含有复杂底物如杂环酯基,芳基氯,酚,醇,炔基,烯基,环丙基,硫等环境中,底物都耐受,也没有钯中毒,收率较高,实在厉害。
操作步骤:To a screw-cap vial was added aryl halide (1.0 equiv), benzaldehyde oxime (1.3 equiv), cesium carbonate (2.2 equiv), and anhydrous DMF (2 mL per mmol of aryl halide). To the resulting mixture was added RockPhos Pd G3 (0.03 equiv). The vessel was sealed and heated at 80 ℃ with rapid stirring for 18 h. For reactions where the cesium salt of benzaldoxime was used (Scheme 5), 2.0 equiv of benzaldoxime was mixed with 2.0 equiv of CsOH-H2O in DMF (1 mL per mmol of benzaldoxime) at 80 °C for 5 minutes during which time a clear yellow solution was formed. Complete deprotonation of the oxime is assumed based on the pKa of benzaldoxime (20 in DMSO) and the pka of water (31 in DMSO). The mixture was cooled to room temperature and the remaining components of the reaction were added and the reaction was carried out as usual. For cases where assay yields were determined, the entire reaction mixture was diluted in a volumetric flask with 3:1 MeCN : 0.1% aqueous H3PO4 and the resulting solution was analyzed on an HPLC instrument calibrated to the product standard. For cases where the product was isolated, the reaction mixture was quenched with 0.1 M HCl and extracted twice with DCM. The combined organic solutions were dried over MgSO4, and purified by silica gel chromatography using a TeleDyne Isco CombiFlash purification system.


 医无忧服务热线:
医无忧服务热线:
